Emission Control for Spark-Ignition Engines
Air pollution:
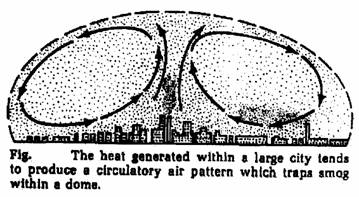
The word smog is coined many years ago form smoke and fog.
|
|
|
Definition:
It is common practice to express the quantity of a gaseous pollutant present in the air as parts per million (ppm). Thus
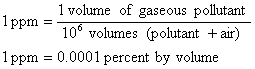
The mass of a pollutant is expressed as micrograms of pollutant per cubic meter of air (μg/m3). Symbolically,
![]()
The basic relation between μg/m3 and ppm at 1 atm and 25° C is
For automobile engines the pollutant is measured in grams per kilowatt per hour (g/kWh).
Also the emission limits is measured by grams per kilometer travel (g/km) or (g/mile).
The carbon dioxide (CO2), water vapor, Oxygen (O2), and Nitrogen (N2) are sometime measured as volume percentage (Vol%).
General listing of air pollutants:
A general classifications of air pollutants is as follows:
1. Particulate matter.
2. Sulfur-containing compounds.
3. Organic compounds.
4. Nitrogen-containing compounds.
5. Carbon monoxide.
6. Halogen compounds.
7. Radioactive compounds.
|
|
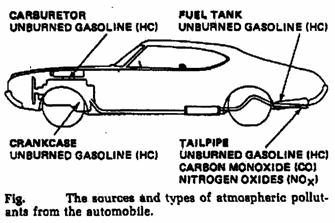
Automobile emission:
Particulates:
The particulates are present in exhaust gases in solid (ash, carbon) or liquid state. One of the most common effects of air pollution is the reduction in visibility resulting from the absorption and scattering of light by airborne liquid and solid materials. Reduction in visibility not only is unpleasing to an individual, but also may have strong psychological effects. In addition, certain safety hazards arise.
In addition to a reduction in visibility caused by particulate air pollutants certain gaseous substances, because of their radiation absorption characteristics, have the potential for causing deterioration in our environment (an increase in the average temperature of the earths surface).
|
|
|
|
Carbon dioxide (CO2) {it is not considered as a pollutant}:
The increase in concentration of carbon dioxide in the atmosphere will lead to increase the earth surface temperature that would melt sufficient ice to cause an increase in the level of the oceans. (Green house effect).
Carbon monoxide (CO)
There is some evidence that CO may be chemically active in smog formation (smoke and fog). Carbon monoxide has long been known to cause death when exposure to a high concentration (> 750 ppm) in encountered. Inhalation of air with a volumetric concentration of 0.3% carbon monoxide can result in death within 30 minutes. The CO-content of the exhaust gas from spark-ignition engines is especially high at idle. It is therefore imperative that the engine never be run in a closed garage.
Sulfur oxides (SOx)
Sulfur oxides generally accelerate metal corrosion. There are several effects of acid rain that are disturbing. First, there is an acidification of natural water sources. This can have a devastating effect on fish life. The demineralization can lead to loss in productivity of crops and forests. Various animal species, including men, respond to sulfur dioxide, (bronchitis).
Hydrocarbons (HC)
Some cancers appear to be caused by exposure to hydrocarbons. Unburned hydrocarbons in combination with the oxides of nitrogen in the presence of sunlight form photochemical oxidants, components of photochemical smog, which do have adverse effects on human health and on plants.
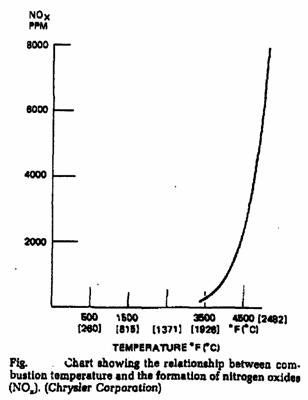
Oxides of Nitrogen (NOx)
Pure NO2 is a poisonous, reddish-brown gas with penetrating odor. NO2 can react with moisture present in the atmosphere to form nitric acid, causes direct damage to materials. It is also responsible to the formation of smog (photochemical oxidants), which are the most damaging to human health.
Effect of pollutants (all of them has the following effects)
o Eye irritation
o Respiratory diseases (old people and small children)
o Reduction in visibility
o Vegetation damage
o Materials corrosion
o Increase the mortality rate
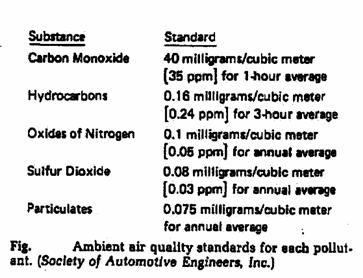
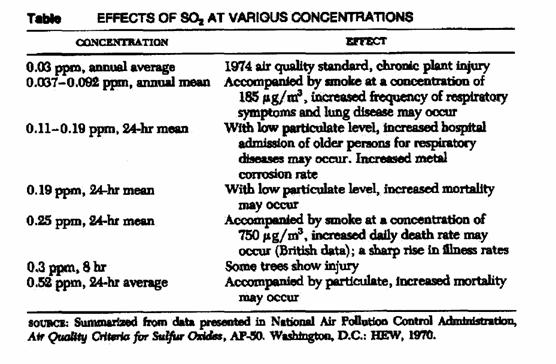
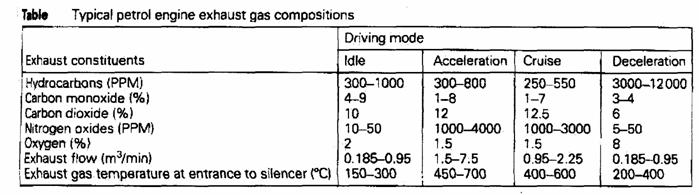
The pollutants concentration (%):
|
|
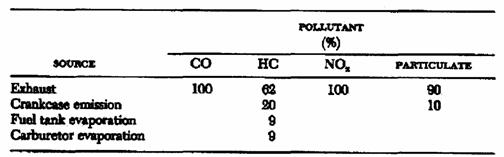
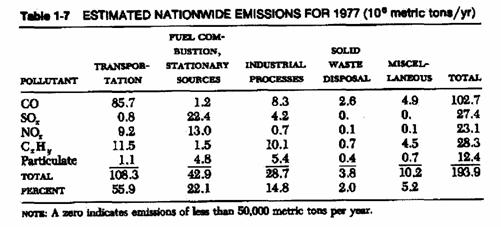
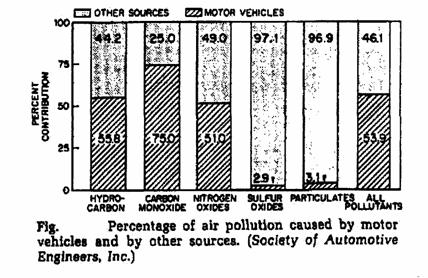
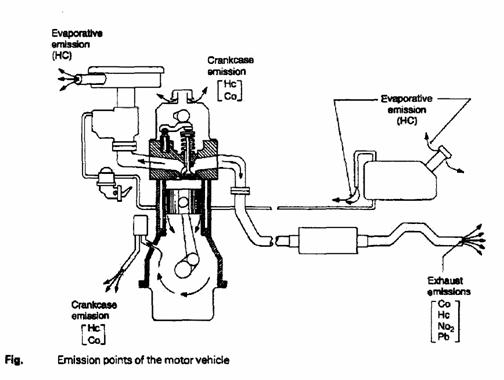
Source of pollution:
|
|
|
|
|
|
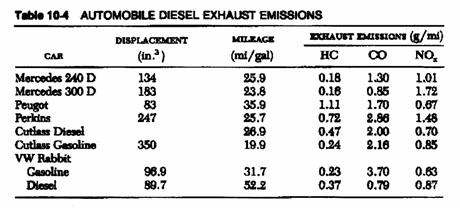
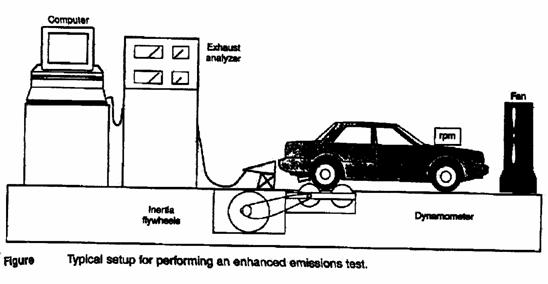
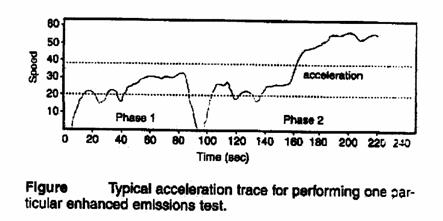
Emission testing:
|
|
|
|
* The emission limit is measured as gram per test (gm/test).
Automobile emission control:
Three basic types of emission control systems are used in modern vehicles; pre-combustion, post-combustion and evaporating control system.
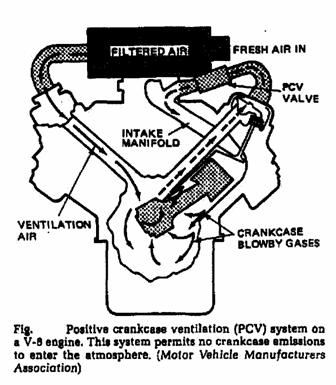
o Pre-combustion control; (Positive Crankcase Ventilation (PCV).
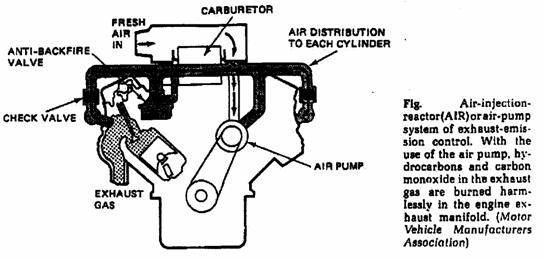
o Engine Modification System; (Secondary air or air injector systems, and Catalytic converters).
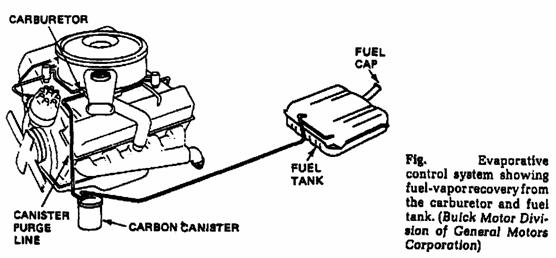
o The evaporative control system; (traps the fuel vapors from fuel tank and carburetor).
Emission control devices:
o Crankcase emission (road draught crankcase ventilation system, Positive crankcase ventilation (PCV) system).
o Fuel evaporation control (fuel tank).
o Air intake temperature.
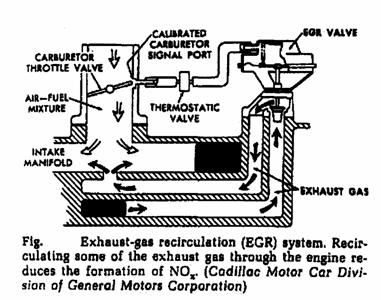
o Exhaust gas recycling.
o Air injected exhaust system.
o Removing the unwanted pollutant gases (catalytic converter).
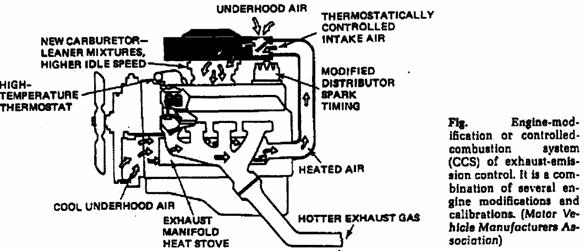
o Engine modifications.
o Alternatively fueled engines.
|
|
|
|
|
Emissions test analyzing
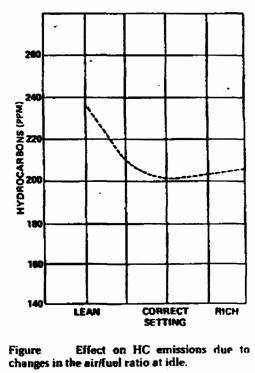
Excessive HC emissions may be caused by:
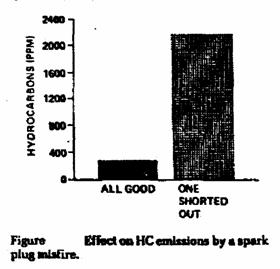
Ignition system misfiring.
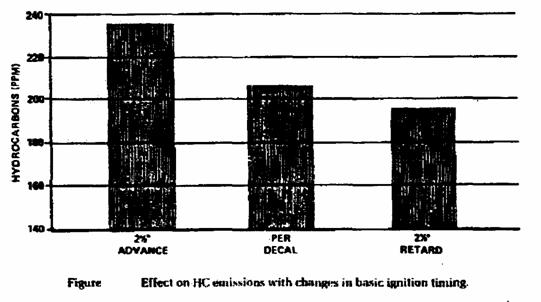
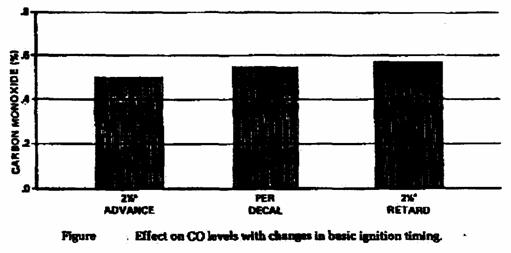
Improper ignition timing.
Excessively lean or rich air/fuel ratio.
Low cylinder compression.
Defective valve, guides, or lifters.
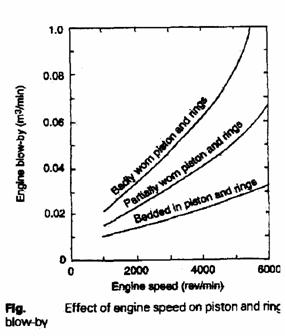
Defective rings, pistons, or cylinders.
Vacuum leaks.
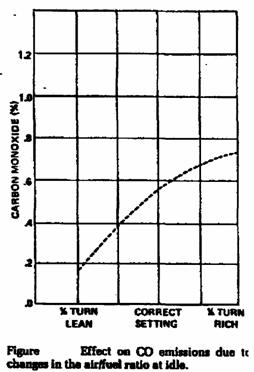
Excessive CO emissions may be caused by:
Rich air/fuel mixtures.
Dirty air filter.
Faulty injectors.
Higher-than-normal fuel pressure.
Defective system input sensor.
Excessive HC and CO emissions may be caused by:
Plugged PCV system.
Excessive rich air/fuel ratio.
Stuck open heat riser valve.
AIR pump inoperative or disconnected.
Engine oil diluted with gasoline.
Lower-than-normal O2 readings may be caused by:
Rich air/fuel mixtures.
Dirty air filter.
Faulty injectors.
Higher-than-normal fuel pressures.
Defective system input sensor.
Restricted PCV system.
Charcoal canister purging at idle and low speeds.
Lower-than-normal CO2 readings may be caused by:
Leaking exhaust system.
Rich air/fuel mixture.
Higher-than-normal O2 readings may be caused by:
An engine misfires.
Lean air/fuel mixtures.
Vacuum leaks.
Lower-than-specified fuel pressures.
Defective fuel injectors.
Defective system input sensor.
* When attempting to identify the exact cause of the abnormal readings, disable the secondary air system. Then rerun the tests. Without the secondary air, the readings will give an accurate look at the air/fuel mixture.
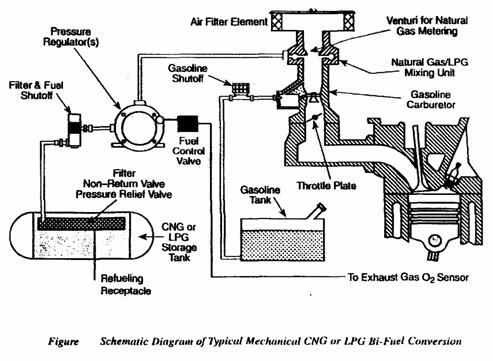
Alternative fuel:
|
|
|
|
|
|
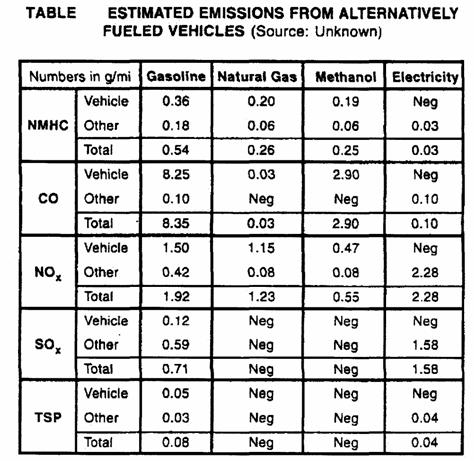
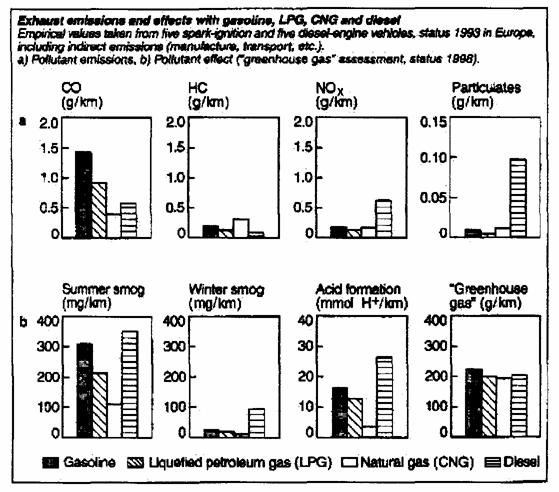
Alternative fuel pollution comparison:
|
|
Operation on alcohol (ethanol and methanol):
Generates lower emissions: reduced NOx and CO2 along with reduced ozone smog formation.
Operation on hydrogen
During combustion, pure hydrogen (H2) oxidizes to form water (H2O). No CO2 is produced by the combustion process. Provided no fossil fuels are used in its production, H2 is thus the only fuel which can be used to avoid CO2.
Electric drive:
Electric drive is the only alternative which can make a similar claim as hydrogen fuel.
* Future NOx emission limits can be met by lean mixtures or a system for catalytic control of emission (still to be developed).
Emission control methods:
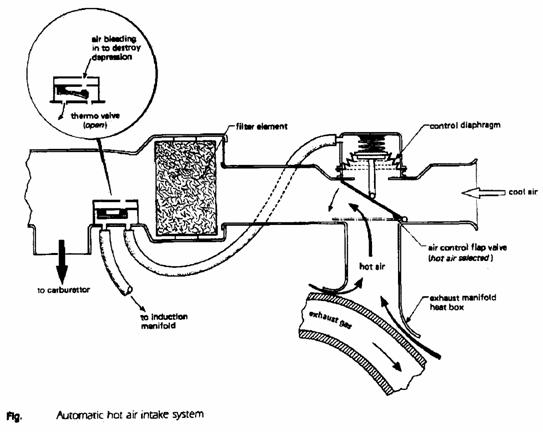
Automatic hot air intake system
The purpose of an air intake temperature control system is to regulate and maintain the temperature of the air entering the induction system within some attainable temperature band (increasing the air temperature will decrease the volumetric efficiency and hence the engine brake power).
|
|
|
|
|
|
|
|
|
|
|
|
Factors influencing combustion chemistry and negative emissions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
https://en.wikipedia.org/wiki/Air_pollution
https://en.wikipedia.org/wiki/Vehicle_emissions_control
https://en.wikipedia.org/wiki/Environmental_impact_of_transport