Thermal Cycle Processes
|
Heat Transfer Solid & Liquid: |
1st Law of Thermodynamics
|
Ideal Gas
|
System
|
|||||
|
Gas: |
||||||||
|
* Const. Volume:
|
* Const. Pressure:
|
|||||||
|
Where: Q = heat, U = internal energy, p = pressure, V= volume, cV = specific heat for constant volume, cp = specific heat for constant pressure, W = work, R= gas constant, T = temperature, hcycle = cycle heat efficiency, Qin = input heat energy, Qout = output heat energy, m = molecular mass, ∆ = change in quantity, g = adiabatic index |
||||||||
|
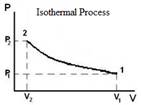
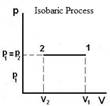
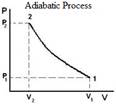
Thermal process |
||||||||
|
Isothermal |
|
Constant Volume |
|
Isobaric |
|
Adiabatic |
|
|
|
T = constant ∆U = 0 |
V = constant W = 0 |
P = constant W = p ∆V |
No heat transfer Q = 0 |
|||||
|
|
|
|
|
|||||