MATERIALS
For most engineering purposes pure metals are too weak and the most important method of increasing their strength, toughness or hardness is by blending them with other metals or non- metals to form alloys.
Often the physical properties of the alloys can be further improved by correct heat treatment, which consists of raising the metal to a required temperature and then cooling at a definite rate.
FERROUS METALS
Ferrous metals are those which contain a large proportion of iron (ferrum), and their varied physical properties depend on the effects of carbon and certain other elements alloyed with the iron.
STEEL
Steel is basically an alloy of iron and not more than l.5 % carbon, which is all chemically combined with the iron. When a greater proportion of carbon is present it cannot all be chemically combined and some will be in the form of free graphite, the metal then being a cast iron.
MILD STEEL
Steel containing up to about 0.25 % carbon is termed mild steel and is very widely used where ductility with reasonable strength and toughness are important, for example in body work, tubing, brackets. Tinplate or ‘tin’ consists of mild-steel sheets with an anti-corrosive thin coating of tin; galvanized iron is also mild steel, with a protective coating of zinc.
THE CARBON STEELS
Medium carbon steel contains from 0.25 % to 0.5 % carbon; with 0.5 % to l.5 % it is usually termed high-carbon steel. Tool or cast steel is a high-carbon steel with 1.0 % or more carbon; silver steel is about a 1 % carbon steel accurately finished to size and polished.
The principal characteristic of the carbon steels is their property of hardening when quenched from a red heat. The hardness and strength increase, but with a loss in ductility, as the proportion of carbon rises to about 1 %. From 1 % to 1.5 % of carbon there is a further increase in the hardness but some reduction in strength of the steel.
ALLOY STEELS
Straight carbon steels are much less employed these days, since the addition of other elements such as tungsten, nickel, chromium, vanadium, molybdenum and cobalt enables special qualities to be imparted to the steel.
High-speed steels, which may contain 0.7% carbon, 18% tungsten, and 5 % chromium with perhaps molybdenum, vanadium, or cobalt, will cut at 5 to 15 times the speed of carbon- steel tools and maintain hardness at a red heat.
During the quenching of large carbon-steel tools, cracks are sometimes produced by the contraction of the metal. This trouble can be avoided by employing an alloy tool steel, a typical example of which contains 1 % carbon, 1 % manganese, 0.75 % chromium, 0.5 % tungsten.
When exceptional strength and toughness are required high- tensile steels are available; these usually contain principally chromium and nickel, with some molybdenum.
Carbon usually remains an essential element in all these alloy steels, and they are nearly always employed in a heat- treated condition to take full advantage of their properties.
THE HEAT TREATMENT OF CARBON STEEL
When carbon steel is quenched from a red heat (about 800 oC) it becomes dead hard and very brittle. For most purposes this brittleness must be reduced and the metal toughened by tempering.
Tempering consists of reheating the metal to a much lower temperature, determined by the intended use of the steel. In the workshop this temperature is often judged by the color of the oxide formed on the surface of the heated metal. For example, a turning tool may be tempered to a straw color— about 225° C.—whilst a spring would need to be raised to a dark blue—about 300o C.—to remove sufficient brittleness and give flexibility.
Annealing is carried out by heating slowly to a red heat (the annealing temperature varies slightly with the carbon content of the steel), ‘soaking’ or maintaining at this temperature for a time d on the size and shape of the article and then cooling as slowly as possible—either inside the furnace or else buried in sand or ashes. This treatment renders the steel soft and more easily machined and also relieves internal stresses which may have been created by cold working or unequal contraction when cooling.
Normalizing consists of cooling from a red heat in air; the rate of cooling is therefore intermediate between quenching,
CARBON-STEEL TEMPERING COLOURS
(of the oxide)
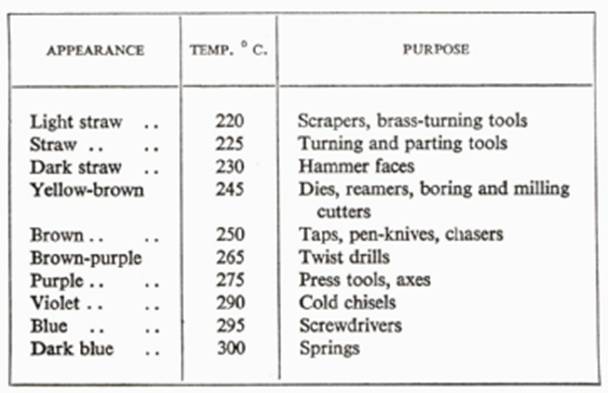
where the object is cooled very rapidly, and annealing, where it is cooled as slowly as possible. Normalizing relieves internal stresses in the steel and refines its structure, which may have been coarsened by prolonged heating, such as is required for some forging operations.
CASE HARDENING
Quenching from a red heat has no appreciable effect on mild steel due to the low carbon content. Steel has the property of absorbing carbon when raised to about 9000 C. to 950° C., and if mild-steel articles are packed in boxes containing charcoal, or other substances rich in carbon, and maintained at this temperature for several hours the carbon content of the surface layers of the metal is considerably increased. This high-carbon ‘case’ can be hardened by quenching in water and, if required, tempered. The resulting article combines a tough mild-steel core with a wear-resisting hardened skin.
In the workshop a quick method of giving a superficial ‘case’ on small mild-steel articles is to heat them to redness and plunge into a suitable hardening powder; the items are then quenched from a red heat. The surface hardness produced by this method is, however, usually limited to some 0.1 mm thick compared with the 1.0 mm or so which can be obtained by the large-scale case-hardening process.
THE HEAT TREATMENT OF ALLOY STEELS
High-speed steels are usually hardened by raising them gradually to about 850° C., then rapidly to a white almost melting heat of about 1,300° C., and then quenching in oil or cooling in a blast of cold air. Secondary hardening by reheating to about 500° C. is often carried out after quenching to further improve the qualities of the steel.
The alloy tool steels and high-tensile steels are generally heat treated by quenching in oil from about 850o C., followed by tempering at a temperature determined by the particular use of the alloy.
For the development of the latent qualities of modern alloy steels and special cast irons, accurate heat treatment is essential, and special furnaces and pyrometers are therefore necessary. For example, many high-speed steels oxidize and scale badly when heated, and a furnace using a controlled atmosphere, from which oxygen is excluded, is employed.
CAST IRON
Cast iron contains from about 2.5 % to 4% carbon together with small quantities of silicon, sulphur, phosphorus and manganese. Grey cast iron is cheap, flows readily into intricate moulds, can be easily machined, and due to presence of free carbon in the form of graphite forms excellent bearing sur faces.
By placing metal chills in the sand mould the hardness of the cast iron can be increased due to the sudden cooling, and this procedure is often employed to produce wear-resisting surfaces.
MALLEABLE CAST IRON
The low tensile strength and brittleness of cast iron can be improved by packing the castings in boxes surrounded by hematite—an iron oxide—and heating for several days at about 900° C. Some of the carbon is oxidized from the castings by this process, and near the surface the material corresponds to mild steel.
SPECIAL CAST IRONS
With the aid of carefully controlled heat treatment and alloys containing nickel, copper, chromium or molybdenum, special cast irons, such as ‘Meehanite’, are now produced which can be used for such highly stressed parts as crankshafts and cam shafts.
These alloy cast irons have the advantages of cheapness, easier machining properties, greater resistance to wear, more uniform density, and an improved vibration damping capa city when compared with the steel forgings which they can replace.
NON-FERROUS METALS
ALUMINIUM
Pure aluminum is a ductile and malleable light metal with a high conductivity of heat and electricity. It resists corrosion by the formation of a surface film of oxide which, if required, can be thickened and hardened by the electrolytic process of anodizing.
With some 4 % copper, 0.5% magnesium, 0.5 % manganese, aluminum forms the ‘duralumin’ alloys used for tubes, bars, sheets, forgings and stampings. These (and several other alloys) have the property of ‘age hardening’—their strength and hardness increase for some days after production, so that with suitable heat treatment duralumin can have a tensile strength three times that of pure aluminum.
Where the strength must be retained at high temperatures, e.g. pistons, other alloys such as the ‘Y’ alloys, containing some 4% copper, 2% nickel, l.5 % magnesium, are employed, usually in the heat-treated condition. More complex aluminum alloys of greater strength and demanding precise heat treatment have been developed, such as RR77, which has a tensile strength as great as carbon steel and on a weight basis is three times as strong. It contains 2.5 %-3.0 % copper, 2 %-4 % magnesium, 4 %-6 % zinc with some silicon, iron, manganese and titanium.
When used for castings aluminum is frequently alloyed with about 12% silicon, which not only increases its strength but makes it run well into moulds.
MAGNESIUM
Magnesium is about 40% lighter than aluminum, and when suitably alloyed offers one of the highest strength-to-weight ratios of ordinary workshop materials. ‘Electron’ crankcases etc. help to improve the power-to-weight ratio of C.I. engines.
A typical composition contains about 8 % aluminum, 0.5% zinc and 0.25 % manganese, and after heat treatment has a tensile strength equal, on a weight basis, to alloy steel.
A further asset of magnesium is the ease with which it can be machined.
COPPER
Copper, one of the few metals used in the pure state, is a very ductile and malleable metal with a very high conductivity of heat and electricity—exceeded only, and to a slight extent, by silver. It is resistant to corrosion, can be easily joined by soldering, brazing, or welding, and forms a great many useful alloys.
BRASS
When copper is alloyed with zinc, brasses are formed; those containing less than 36% zinc are widely used for cold working. Above this proportion harder and stronger brasses are obtained which are usually worked hot.
Brass can be strengthened by the addition of aluminum, tin, iron, and manganese to form the ‘high-tensile brasses’.
BRONZE
Bronze was originally an alloy of copper and tin, but the word has come to be used for copper alloys containing no tin. ‘Gunmetal’, frequently used for corrosion-resisting castings, contains about 10% tin and 2% zinc. Phosphor bronze usually has about 10% tin with 0.5 % Phosphorus. An important series of copper alloys is the aluminum ‘bronzes’ containing from 6% to 11 % aluminum. Those with the higher proportions often have additions of iron and nickel and can have their strength considerably increased by heat treatment.
ZINC
Pure zinc is a rather brittle corrosion-resisting metal, and used for this latter property in processes like galvanizing, ‘Brylanizing’, ‘Sherardizing’, etc.
Zinc alloys used for pressure die-casting contain some 4% aluminum, 3 % copper and 0.02 % magnesium, and are therefore known as ‘Mazak’ (‘k’ for copper).